Global Orthopedic Market – Industry Overview in Brief
The worldwide orthopedic market was approximately $56.5 billion in 2023 with orthopedic implants accounting for $48 billion and instruments and equipment representing the remaining $8.5 billion. In 2023, the orthopedic implants market increased 7% to $47.9 billion as many of the large companies generated above average sales growth due to healthy patient demand, improvements in hospitals’ staffing levels, some backlog and minimal COVID impacts.
Looking forward, we project growth of 4% annually over the next several years, absent any unforeseen catastrophic events, as mid-single digit growth in procedures is offset by low-single digit price declines.
In this whitepaper, we include 2023 revenue from the major manufacturers versus 2022.
We list the largest manufacturers in the global orthopedic market below:
Table 1

Table 2

Globus Medical and NuVasive merger
In 2023, the orthopedic market experienced healthy patient volumes along with new product launches by many companies. One key notable event was the merger of Globus Medical and NuVasive on September 1, 2023, which created a strong #2 company in the $10+ billion global spine market with pro-forma revenue of $2.4 billion. With its increased global scale, expanded commercial presence, and expanded product, services and technology portfolio, Globus Medical will operate from a position of commercial and financial strength, enabling it to take further market share over the next few years.
2023: Notable Events and Trends
2023 was a robust year for the medical device sector due to above average patient volumes and managable challenges. We highlight some events and trends specific to the orthopedic sector:
· The orthopedic market experienced above average procedures volumes as patients’ demand was healthy (aging demographics, physical activity levels, increased ASC volume) and a portion of the COVID backlog was completed. International markets also experienced healthy volumes and grew relatively in-line with US markets. Foreign currency exchanges rates had minimal impact of 1% or less on revenue in 2023.
· Most management believe there is a substantial backlog of elective procedures, and this backlog will be worked down gradually over time; the timing of when these procedures will be completed remains uncertain. Some management believe the backlog will not be overly material in any given year as it will take years to work through it given capacity constraints.
· Inflation and some supply chain still impacted costs of good and operating expenses for most companies in 2023. Most companies are working diligently to offset these higher expenses, so they have a lesser impact on earnings. Orthopedic companies are working with their hospital customers on pricing to have a less negative impact going forward.
· COVID accelerated the trend towards Ambulatory Surgical Centers (ASCs) from hospital settings and this trend continues as ASC procedure volume is growing every year. Orthopedic companies are offering more products and services to accommodate the ASC setting as every hospital system is constructing ASCs. Stryker estimates that 12-15% of its large joints procedures were done in ASCs in 2023, up from 10% in 2022.
· Robotic systems for joint replacement surgeries experienced solid demand throughout 2023. Robotic system adoption for orthopedics continues at a steady pace with more than 20% of operating rooms having access to a robot. Companies continue to invest in R&D in robotics to launch different applications for robotic systems with Zimmer receiving FDA approval for its shoulder application in February 2024 with commercial launch expected in second half of 2024. Stryker expects to launch its shoulder application in late 2024.
· Orthopedic companies are investing in technology, software, robotics, and data informatics to provide surgeons with data and information to achieve better and more predictable surgical outcomes for their patients.
· Within orthopedics, there have been several announced M&A transactions in 2023 and early 2024. Many companies are merging to increase scale with their sales organizations, fill gaps in their product portfolios, and realize cross-selling opportunities, cost savings and economies of scale.
– January 2023: Orthofix and SeaSpine completed their merger to create a leading spine and orthopedic company with a combined $747 million of revenue in 2023 with $419 million in spine implants/biologics and $213 million in bone growth stimulation. As these two companies had small market share positions in the spine market, the combined company has ~6% market share in the $10+ billion global spine market.
– September 2023: Globus Medical and NuVasive completed their merger to create a global spine and musculoskeletal company with $2.4 billion of pro-forma revenue in 2023. This transaction created a solid #2 company in the global spine market with more than 20% market share behind #1 Medtronic. This transaction will generate significant scale, expand its commercial presence, and create a robust and innovative product portfolio, enabling the new company to take further market share. With its main focus on the spine market, the combined company could be the preeminent spine company, if the integration goes well, with its continuous new product innovations, broader commercial reach backed by its financial strength.
– January 2024: Enovis, an orthopedic and rehab/soft goods company, acquired LimaCorporate for 800 million Euros with ~ $300 million of revenue. With $1.7 billion of revenue in 2023, Enovis will augment its reconstructive segment revenue from $630 million in 2023 to approximately $1.0 billion in 2024 with this transaction. This deal brings commercial scale and expands product portfolio to its reconstruction segment.
· Despite some market share gains by smaller companies during the COVID years, market share did not materially change since 2020 as most large orthopedic companies experienced similar revenue percentage changes, absent acquisitions, in each specific orthopedic segment. Some companies with robotic offerings in specific segments have gained market share by 1-2% over the past three years due to higher implant pull-through. In general, market share shifts move gradually in the orthopedic market, hence, many companies turn to M&A to scale up via consolidation.
We layout specific segments within the orthopedic market and market share positions as follows:
Knee and Hip Market
The 2023 worldwide knee and hip market generated $16.5 billion in sales, which is an increase of 7.5% from 2022 levels. The knee and hip implant markets were $9.0 billion and $7.5 billion, respectively, with knee implants up ~10% and hip implants up 5%. We believe that knee procedures are growing faster as they were the first type of reconstructive surgeries to be done on robotic systems, followed by hip implants. Stryker noted that 60% of its US knees are done on its MAKO surgical robots vs. 34% of its US hips exiting 2023. We expect both the knee and hips market to perform well in 2024 as hospitals are managing capacity effectively with some backlog being work down. In 2023, price declined by 1% within the range of historical levels.
The top-four companies dominate these two segments with approximately 80%+ share. In the knee market, Zimmer Biomet, Stryker and JNJ DePuy are the top-three market leaders with a 34%, 29% and 16% share, respectively, followed by Smith & Nephew with 11% share. In the hip market, Zimmer Biomet, Stryker and JNJ DePuy are the top-three market leaders with a 26%, 23% and 21% share, respectively, followed by Smith & Nephew with 9% share.
Table 3

Knees and Hips – Robotic Systems
The biggest trend in the knee and hip implant market is the rollout of robotic assisted surgery systems by all the major manufacturers.
· In late 2013, Stryker had the first mover advantage when it acquired MAKO Surgical and now has approximately 2,000 systems installed worldwide at year-end 2023. Stryker’s MAKO system is also approved for total hip applications in addition to total knee and partial knee. Stryker expects to launch its spine application on its MAKO system in Q3 2024 and its shoulder application in late 2024.
· In 2020, Smith & Nephew launched its CORI surgical system, a handheld robotic solution, for its knee implants and in early 2022 for total hip implants. As of year-end 2023, there are close to 800 CORI systems installed. In the third quarter of 2023, the company added a robotic revision knee application with its CORI system, which is the first revision knee robotic application on the market.
· In 2019, Zimmer Biomet launched its ROSA knee system for robotic-assisted knee replacement surgeries. We estimate there are more than 1,100 systems placed at the end of 2023. In 2021, Zimmer rolled out its partial knee and total hip application for its ROSA system. Zimmer is expected to launch its shoulder application in the second half of 2024.
· In early 2021, JNJ DePuy Synthes received FDA approval for its VELYS robotic system for total knee and received CE mark in 2023.
With the four large manufacturers having robotic assisted-surgery system offerings, the adoption of robotic for joint replacement surgeries will continue for the foreseeable future given that more than 20% of operating rooms have access to a robotic system. Additionally, many of these companies are expecting to roll out spine, shoulder and/or other applications for their robotic systems over the next 1-2 years.
Spine
The 2023 worldwide spine market generated $10.5 billion in sales, an increase of 6.6% from 2022. The top four spine companies currently control close to 80% of the market due to ongoing consolidation, including the merger of Globus Medical and NuVasive in 2023. This segment is dominated by Medtronic with 32% market share, followed by Globus Medical with an approximate 23% share. Beyond Medtronic and Globus Medical, the spine market is relatively fragmented with many companies having 12% market share or less. We provided details on the spine transactions in 2023 and early 2024.
Table 4

· In January 2023, Orthofix and SeaSpine merged to create a leading spine and orthopedic company with a combined $747 million of revenue in 2023 with $630 million in spine implants/biologics. As these two companies had small market share positions in the spine market, the combined company has ~6% market share of the global spine market.
· In September 2023, Globus Medical and NuVasive closed on its merger to create a global spine and musculoskeletal company with $2.4 billion of pro-forma revenue in 2023. This transaction will create a solid #2 company in the global spine company with 20%+ market share behind #1 Medtronic.
· In April 2024, ZimVie sold its spine segment to investment firm H.I.G. Capital for $375 million. This segment generated $409 million of revenue in 2023. Since this is the only spine asset for ZimVie and its new owner, H.I.G. Capital, this transaction does not have impact on the global spine market’s share positions.
We expect the spine market to grow at a 3% CAGR over the next few years. In the US, mid-single digit growth in procedures has been offset by low-single digit price declines. The robotic trend is experiencing increased adoption in the spine market with Medtronic’s Mazor system and Globus Medical’s Excelsius system. Stryker and JNJ are also working to bring their robotic spine applications to market.
Trauma/Fixation
The 2023 worldwide trauma market generated $6.2 billion of sales, up 4.7% from 2022. This segment is driven by traffic accidents, violence, falls and other accidents. Unlike other orthopedic segments, the trauma market is not necessarily associated with aging demographics and its procedures are not necessarily elective, and often require immediate care.
Table 5

This segment is dominated by JNJ DePuy which acquired Synthes in June 2012 for $19.7 billion. As a result of this acquisition, JNJ has a 45%+ market share of the worldwide trauma market. Stryker has emerged as a solid number 2 company in the trauma market over the past few years via small tuck-in acquisitions and new product introductions.
The trauma market is growing low-single digit on an annual basis. Volume and mix are the main drivers of growth offset by pricing pressure. We expect the trauma market to grow at a 3% CAGR over the next several years.
Sports Medicine
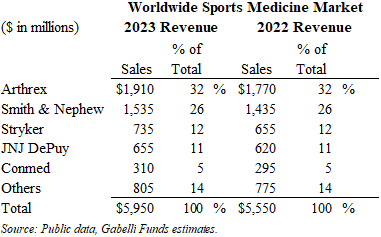
The 2023 worldwide sports medicine market generated approximately $6.0 billion of sales, up 7% from 2022. This segment is driven by injuries related to sports and exercise. Given that many sports injury procedures are done in surgery centers (ASCs) and away from the hospital setting, these procedures are growing at a healthy pace.
The market share leader is privately-held Arthrex with an estimated 32% share, followed by Smith & Nephew at 26%, which includes its May 2014 acquisition of ArthroCare. Stryker and JNJ DePuy have the #3 and #4 market share positions with 12% and 11% share, respectively.
Table 6
Extremities
The 2023 worldwide extremities market generated $5.5 billion of sales, an increase of 9% from 2022. This segment is experiencing the fastest growth due to new product innovations that are allowing surgeons to treat conditions that were previously often untreatable. This market is typically segmented into upper extremity (shoulder, elbow, hand) and lower extremity (foot and ankle) as orthopedic surgeons treat the former and podiatrists and orthopedic surgeons treat the latter. Both large and small orthopedic companies compete effectively in this segment, whose growth is largely driven by innovation.
In November 2020, Stryker completed its acquisition of Wright Medical, becoming the #1 market share leader with 30% share. The remainder of this segment is relatively fragmented since innovation drives adoption and an innovative product line can drive sales growth for a new entrant.
Table 7

We expect the global extremities market to grow high-single digits CAGR over the next few years. This market will be driven by new product innovation, which expands the market opportunity as more patients are able to be treated. Additionally, the introduction of robotic assisted system for shoulder applications by both Zimmer Biomet and Stryker in the second half of 2023 will make the procedure more efficient for the surgical team.
GLP-1 Impacts on Orthopedics/ Reconstructive Surgery
Most orthopedic companies providing implants and services for joint reconstructive surgery believe that GLP-1 drugs will have a modest positive impact on their businesses in the short term and long term. In the short term, patients who were not currently surgical candidates due to high BMI are becoming eligible candidates due to their weight loss. Some doctors are not comfortable performing surgeries on candidates with BMI greater than 30 in some countries. In the long run, weight loss leads to more active physical activity and increased longevity, which could result in more surgeries.
The primary reason for reconstructive surgeries is osteoarthritis, which is caused by age, genetics, joint injuries and other factors. Osteoarthritis occurs when the cartilage between joints breaks down and impacts the structure and function of the joint. Even though weight loss can alleviate some osteoarthritis pain and slow its progression, it cannot reverse osteoarthritis.
Consolidation in the Orthopedic Market
There is ongoing consolidation in the orthopedic market as scale and breadth of product portfolio are critical for success. Additionally, there has been ongoing consolidation among hospitals due to economic pressure, thus resulting in aggregating purchasing decisions, which in turn limits suppliers and lowers price. As hospitals work with a limited number of orthopedic manufacturers, they prefer manufacturers with broad product portfolios and diversified product lines. In 2023, there was one transaction above $2.0 billion and various smaller transactions.
We list the major orthopedic transactions since 2012 below. There are numerous other transactions of smaller, tuck-in product lines of small, private companies that are not listed. In general, the median revenue multiple paid was slightly less than 4.0x. For companies generating earnings, the median EBITDA multiple paid is approximately 12x. Products that are highly differentiated and/or growing at above market growth rates tend to command higher multiples.
Table 8 Orthopedic Transactions

In 2023, the global spine market consolidated even more with the merger of Orthofix and SeaSpine in January 2023 and the combination of NuVasive and Globus Medical in September 2023. Consolidation in the spine market makes sense as this segment was extremely fragmented. Furthermore, Enovis acquired LimaCorporate to augment its reconstructive segment and gain scale in January 2024.
Although we believe further consolidation will occur within orthopedics over the next few years, we also expect orthopedic companies to acquire companies to expand digital and technology offerings that complem
nt their current product portfolio. Orthopedic companies are offering more data-driven solutions and service offerings to customers to enable improved solutions and outcomes.
Exhibit 1

We highlight orthopedic companies that could participate in ongoing consolidation trend, either as acquirers or targets, on the following pages.
Table 9 Orthopedics Grid


COMPANY OVERVIEW
Headquartered in Kalamazoo, MI, Stryker is one of the world’s leading medical technology companies with $20.5 billion of revenues in 2023. Approximately 42% of its revenues are from products in the orthopedic market where Stryker sells knee, hip, trauma, extremities and spine implants and related products, where it has leading market positions. Approximately 44% of revenues are in the MedSurg segment where Stryker sells instruments, endoscopes, medical beds/stretchers and other products and services to hospitals. The remaining 14% of its revenues are from its neurotechnology segment. Approximately 74% of its revenues are derived from the US and 26% outside the US. Stryker has been active in acquisitions over the past decade with its most recent acquisition of Vocera Communications for $3.0 billion in February 2022.
As M&A is a key component of its strategy to increase shareholder value, Stryker will continue to make strategic acquisitions (mostly tuck-ins). We highlight some key business and financial aspects:
· In 2023, Stryker’s revenue grew 11.5% organically to $20.5 billion. Its sales growth was broad based as its orthopedic and spine segments grew 11.1% while MedSurg and Neurotech segments grew 11.8%.
· In 2023, most of its ten business segments grew revenue between 8.8% to 14.4% in constant currency except its spine and neurovascular segments, both of which grew 4.0%.
· In 2023, Stryker benefited from healthy patient demand with favorable demographics and active lifestyles, some COVID backlog, hospitals operating surgeries at full capacity, and growing ASC capacity.
· In 2024, Stryker expects to grow its organic sales at 7.5-9.0% based on strong procedure volumes and a stabilizing macro-economic environment.
· With regards to its MedSurg segments, capital demand from its hospital customer remains healthy and Stryker exited 2023 with a higher backlog for capital equipment, which bodes well for its MedSurg segments.
· In 2023, Stryker launched a variety of new products in its various segments, and it expects 2024 to be another year of big product launches, including its shoulder application on its MAKO robotic assisted system.
· At year-end 2023, Stryker’s net debt to EBITDA was less than 2.0x, providing the company the financial flexibility for strategic M&A.
· Past acquisitions include:
– February 2022 – Acquisition of Vocera Communications for $3.0 billion to enter into the global digital care coordination and communications market, a $3-5 billion total addressable market (TAM), to augment its advanced digital healthcare offerings.
– November 2020 – Acquistion of Wright Medical for $5.6 billion to gain #1 market position (30% share) in the ~$5.5 billion global extremity market; this acquisition strengthened Stryker’s postion in the lower extremities (foot/ankle), but more so in the upper extremities (shoulder) where Stryker had a small presence.
· Similar to other orthopedic companies, Stryker is still experiencing pricing headwinds in its orthopedic segments with price declines of 1% annually despite cost inflation. Stryker is experiencing some price power in its MedSurg segments given its steady cadence of new product introductions.
Stryker expects to be active in strategic M&A in 2024 given that it paid down some of its debt in 2023 and currently has ample financial flexibility. Stryker’s M&A strategy has been consistent over the past decade as it has focused on transactions in current or adjacent segments that can leverage Stryker’s existing commercial infrastructure to drive sales growth.

COMPANY OVERVIEW
Headquartered in Warsaw, Indiana, Zimmer Biomet Holdings is the second-largest orthopedic implant company in the world. With $7.4 billion of revenue in 2023, Zimmer is the largest global knee and hip implant manufacturer with approximately 33% market share. Its main products are knee implants, hip implants, sports medicine products, trauma/extremities implants, and surgical products. Zimmer generated 59% of revenue from the Americas and 41% from international markets in 2023. On March 1, 2022, Zimmer Biomet spun-off its dental and spine segments into ZimVie (“ZIMV”).
As the largest company in the global knee and hip implant market, Zimmer continues to shape its product portfolio to drive growth. We highlight some key business and financial aspects:
· In 2023, Zimmer’s revenue increased 7.5% organically to $7.4 billion as its segments performed well with some COVID backlog being performed. Management believes there is a huge backlog of elective procedures, but the timing of those surgeries remains uncertain.
· In August 2023, Zimmer named Ivan Tornos as its new CEO after having served as its COO since March 2021. He joined Zimmer Biomet in November 2018 and previously worked for various medical companies for more than 20 years.
· In 2024, management expects sales to grow 5-6% in constant currency with healthy patient demand and solid procedure volume.
· In 2024, management does not expect any supply constraints to hamper sales growth as it is in a stable supply environment. Supply challenges were a headwind to sales growth in 2022 and 2023.
· In 2019, Zimmer Biomet launched its ROSA knee system for robotic-assisted knee replacement surgeries. In 2021, Zimmer launched ROSA system for its partial knee and total hip applications. This system, along with new product launches, should enable Zimmer to grow above market growth in the US over the next few years. There were more than 1,000 systems placed at year-end 2023. Zimmer expects to launch its the shoulder application for its Rosa robotic system in the second half of 2023.
· Zimmer continues to introduce new products in its segments and expects to launch 40+ new products in the next two years. In early 2024, Zimmer will fully launch its Persona® OsseoTi® cementless knee system, its Persona IQ and three large hip products.
· Similar to other orthopedic companies, Zimmer is investing in its ambulatory surgical center (ASC) infrastructure to expand its ASC business given the expected growth over the next few years.
· Management expects operating margin improvement over the long term as it makes targeted investments to drive sales growth.
· Zimmer’s net debt to EBITDA is approximately 2.1x; it currently has the financial flexibility to make strategic M&A to re-shape its portfolio.
· In early 2023, Zimmer acquired privately held Embody, a medical company focused on soft tissue healing, for $155 million plus $120 million in payments for potential milestones. For the remainder of 2023, Zimmer acquired three small private companies totaling $118 million in upfront payments and $68 million in milestone payments.
We expect Zimmer to be active in strategic M&A transactions, mostly tuck-ins, in the near term. The company is focused on re-shaping its portfolio to accelerate growth for its core knee, hip and SET segments.

COMPANY OVERVIEW
Headquartered in London, UK, Smith & Nephew is a global medical technology company, distributing products and solutions for joint reconstruction, advanced wound management, sports medicine and trauma and extremities. The company generated $5.55 billion of revenue in 2023. It is the fourth-largest orthopedic company in the global orthopedic market. Its revenue consists of 30% from joint reconstruction, 29% advanced wound products, 28% sports medicine/arthroscopy, 10% trauma/extremities and 3% ENT.
Smith & Nephew is the fourth largest orthopedic company in the world. We highlight some key business and financial aspects:
· In 2023, revenue increased 7.2% in constant currency to $5.55 billion with sports medicine up 10%, advanced wound management up 6.4% and orthopedics up 5.7%.
· Solid growth across all three business segments was driven by improved sales execution, new product launches, and improved product availability.
· In July 2022, management announced a 12-Point Plan to transform to a higher growth company. This plan includes improving commercial execution with continuous new product launches, improving operational systems, overhauling supply chain and driving efficiency.
· During 2023, the company made progress with its plan with the following: improving its commercial strategy in all three segments, fixing supply chain in its orthopedics segment, improving its cost structure, implementing portfolio pricing strategies, with continued focus on R&D and innovation. Its 2025 adjusted operating margin target goal of at least 20% remains unchanged.
· Key franchise areas include the following:
– Sports Medicine & ENT (31% of revenue) – Broad array of instruments, implants and technologies for minimally invasive repair of knees, hips and shoulder repairs; high-definition cameras, digital image capture, scopes, light sources and monitors, RF probes, electromechanical and mechanical blades.
– Orthopedic Reconstruction (Knees/Hips) (30% of revenue) – Knee systems include JOURNEY II with OXINIUM, LEGION and ENGAGE. Hip systems include the POLAR3, OR30, ANTHOLOGY, the R3 System, and REDAPT revision; CORI surgical systems with robotic assist.
– Advanced Wound Management (29% of revenue) – Products for the treatment of acute and chronic wounds, including leg, diabetic and pressure ulcers, burns and post-operative wounds; traditional and single-use negative pressure wound therapy (‘NPWT’) and hydrosurgery systems.
– Trauma & Extremities (10% of revenue) – Products include plates and screws, intramedullary nails, hip fracture, limb restoration, extremities and shoulder replacement.
· The company continues to make small acquisitions with products that strategically fit its portfolio. In January 2024, the company acquired CartiHeal for a novel sports medicine technology for cartilage regeneration in knees for $180 million upfront plus $150 million in contingent payments.
· In any given year, the company generates approximately $1 billion of annual operating cash flow, re-invests approximately $400 million for capex and instrument sets, pays a dividend and makes strategic acquisitions. In 2023, the company spent an incremental $300 million on working capital.
We believe Smith & Nephew is a possible acquisition candidate for a large medical company. It has an approximate 25% share in the global sports medicine market, approximate 15% share in the advanced wound care market and an approximate 10% market share in the global knee and hip implant market.

COMPANY OVERVIEW
Headquartered in Audubon, PA, Globus Medical, founded in 2003, is the second largest spine company in the $10+ billion global spine market. Globus Medical groups its products and services into two segments: Musculoskeletal Solutions and Enabling Technologies. Its Musculoskeletal Solutions segment (90%+ of revenues) includes a comprehensive spine portfolio for treatment of various spine disorders and pathologies, orthopedic trauma solutions to treat a variety of traumatic injuries and fractures and hip and knee joint solutions. Its Enabling Technologies products (<10% of revenues) include computer assisted intelligent systems consisting of imaging, navigation and robotics (INR) solutions to enhance surgeons’ capabilities to improve patient outcomes.
On September 1, 2023, Globus Medical and NuVasive completed their merger to create a global spine and musculoskeletal company with $2.4 billion of pro-forma revenue in 2023. This transaction created a solid #2 company in the global spine company with 20%+ market share behind #1 Medtronic.
Globus Medical is currently one of two companies with a robotic system spine offering. We highlight some key business and financial aspects:
· Second largest spine company in the world behind #1 Medtronic.
· In 2023, Globus Medical stand-alone grew sales 13% organically driven by new product launches, sales execution, and robotic implant pull-through.
· In 2023, Globus generated approximately $120 million from its Excelsius GPS™ system and other enabling technologies; Over 65,000 procedures have been done using Excelsius technology since launch.
· With the NuVasive merger, Globus Medical has doubled its US spine sales team, bringing over 350 reps.
· In any given year, management’s goal is for the company to introduce 10-15 new products in spine.
· 2023 was the fifth year for Globus in the global $6.0 billion trauma market; Globus continues to invest in its trauma business with new product launches, instrument set investments and new sales rep hires.
· With its continuous investment in robotics and trauma, Globus is expected to grow above market rate for the foreseeable future.
· With the NuVasive merger, Globus Medical’s combined 2024 EBITDA margin is expected to be 29-30%. As Globus expects to generate $170 million of cost synergies from 2024-2026, its EBITDA margin is expected to return to its historical 33-35% EBITDA margin by end of 2026.
· Globus generated free cash flow of $165 million in 2023; we expect management to remain focused on the NuVasive merger integration activities over the next 2-3 years.
· International sales represented 18% of 2023 net revenue, which is expected to grow as Globus is underleveraged in certain international markets.
Globus Medical continues to invest in many areas in addition to broadening its robotic platform and expanding its application in various orthopedic procedures. Although management is focused on the NuVasive merger integration over the next 2-3 years, Globus Medical has the financial flexibility for smaller, strategic tuck-in acquisitions.

COMPANY OVERVIEW
Headquartered in Wilmington, DE, Enovis is a manufacturer and distributor of orthopedic rehab products (Prevention & Recovery – P&R segment) and Reconstructive surgical products and services (Recon segment). The company was formed in April 2022 when its predecessor company, Colfax, spun-off its ESAB industrial segment into an independent public company. With the spin-off, the remaining Enovis consisted of its DJO Global business and small tuck-in reconstructive acquisitions generating $1.7 billion of revenue in 2023 with two segments: P&R products (63% of revenue) and Recon products and services (37% of revenue). Its P&R products include rigid bracing products, orthopedic soft goods, vascular systems and compression garments, hot and cold therapy products and bone growth stimulators and electrical stimulators. Its Recon products include knee, hip, shoulder, elbow, foot, ankle, and finger implant products and surgical productivity solutions.
On January 4, 2024, Enovis acquired LimaCorporate, a European orthopedic company with ~ $300 million of revenue, for €800 million. This acquisition will increase Enovis’s Recon segment revenue from an estimated $700 million to $1.0 billion in 2024 with approximately 50% of revenue from extremities and 50% from knees and hips. Enovis’s strategy is to continue to grow and scale its reconstructive portfolio over time.
Enovis is evolving itself via strategic tuck-in acquisitions to compete in the global orthopedic market. We highlight some key business and financial aspects:
· With an estimated $1.0 billion of revenue in its Recon segment in 2024, Enovis is gaining scale to improve its competitive positioning in the extremity market and knee/hip markets.
· With the LimaCorporate acquisition, Enovis becomes a Top 3 company in the $5.5 billion global extremity market with approximately 8% market share; it has a greater presence in the shoulder market than foot/ ankle.
· Its LimaCorporate acquisition accelerates its progress toward its strategic goals: high-single digit organic sales growth, continued margin expansion and global scale.
· In 2023, Enovis grew sales 8% organically driven by double-digit growth in its Recon segment and mid-single digit growth in its P&R segment. This growth was driven by new product launches and solid execution.
· In 2024, Enovis expects ~7% organic sales growth with double-digit sales growth from its Recon segment and low-to-mid single digit sales growth from its P&R segment along with more than 50bps margin expansion.
· With its new product launches and increased global scale, Enovis expects to grow above market rate and gain market share in 2024.
· Enovis utilizes its proprietary Enovis Growth Excellence (EGX) business system to drive continuous operational productivity and pricing improvements.
· With its LimaCorporate acquisition, Enovis’s net debt to EBITDA is 4.5x; the company is focused on integration of Lima during 2024 while it deleverages for potential acquisitions in 2025 and beyond.
· International sales represented 32% of 2023 net revenue, which will grow to 40% in 2024 inclusive of LimaCorporate’s revenue contribution.
Enovis continues to scale its reconstruction segment via strategic tuck-in acquisitions. As it integrates these acquisitions, the company continues to drive operational improvements. As its market share in both the extremity market and knee/hip markets are relatively low (< 10% share), the company will continue to look for strategic acquisitions to increase its scale over time.
.

COMPANY OVERVIEW
Headquartered in Largo FL, Conmed is a manufacturer and marketer of products for orthopedic surgery and general surgery. Its general surgery products (57% of revenue) include its AirSeal insufflation management system, Buffalo filter smoke evaluation products, electrosurgical and endomechanical products, and endoscopic products. Its orthopedic surgery products (43% of revenue) include sports medicine repair products, allograft tissue, powered surgical instruments, and visualization products. In the US, Conmed sells its products using its direct sales reps for general surgery and a hybrid sales model for orthopedics. It generates 44% of its net sales from international markets. The company generated $1.24 billion of revenue in 2023.
We highlight some key business and financial aspects:
· In 2023, Conmed’s revenue grew 12% to $1.24 billion, or 4.6% adjusted for warehouse issue, due to strength of its product portfolio and solid sales execution.
· In the fourth quarter of 2022, sales were impacted by a warehouse software management implementation in October, which impacted revenue by $65 million with general surgery segment more impacted than orthopedics.
· In the past few years, Conmed has evolved its product portfolio toward a greater mix of high growth, high margin product offerings.
· In 2023, approximately 83% of its revenue are single-use, recurring revenue.
· Main products include the following:
– Sports medicine and allograft- Arthroscopes, tissue repair sets, suture anchors, metal and bioresorbable implants; Includes BioBrace®, TruShot® soft tissue fixation system, Y-Knot® all-suture anchors, and Argo™ knotless suture anchors.
– Powered instruments- Hall surgical brand name for large and small bone procedures, trauma.
– Surgical visualization- 2DHD and 3DHD vision technologies for general surgery and MIS surgeries.
– AirSeal – Insufflation system for stable pneumoperitoneum, constant smoke evacuation, and valve-free access to the abdominal cavity during surgery.
– Buffalo Filter- Surgical smoke evaluation pencils, evacuators, filters and accessories.
– Electrosurgical offerings- Monopolar and bipolar generators, argon beam coagulation generators, handpieces, smoke management systems and other.
– Endomechanical offerings- Full line of instruments, including the Anchor1 line of tissue retrieval bags, trocars, suction irrigation devices, graspers, scissors, and dissectors.
– Endoscopic offerings- Minimally invasive diagnostic and therapeutic products used in conjunction with gastroenterology procedures which utilize flexible endoscopy.
· With its cashflow generation in 2023, Conmed was able to bring its net debt to EBITDA to 4.4x.
We believe Conmed is a possible acquisition candidate for any company who would like to expand its presence in sports medicine and general surgery and strengthen its international presence.

COMPANY OVERVIEW
Headquartered in Carlsbad, CA, Alphatec is a manufacturer and marketer of implants, biologics and systems for the treatment of various spine disorders. The company’s spine portfolio consists of access systems, fixation systems, interbody systems, biologics, neuromonitoring and imaging systems. The company markets its products using strategic independent distributors and direct sales reps in the US. The company generated $482 million of revenue in 2023, up from $92 million in 2018. International revenue was 8% of revenue in 2023.
We highlight some key business and financial aspects:
· In 2023, revenue increased 38% to $482 million as the company continues to gain market share by launching new products and attracting new surgeon customers, esp. in lateral procedures. For the past five years, the company has grown its revenue significantly and expanded its market share in the global spine market.
· In 2023, the company launched 15 new products and product extensions and trained more than 500 surgeons.
· Its main products include the following:
- Spinal technologies and systems – Includes PTP patient positioning system, Sigma access systems, Calibrate™ PSX system, InVictus fixation systems, IdentiTi implants, spinal implants made from allograft, PEEK and porous titanium with NanoTec surface modifications.
– Biologics – Includes allograft spacers, 3D ProFuse Bioscaffold, family of AlphaGRAFT products (DBM, CBM), and Amnioshield amniotic tissue barrier.
– SafeOp neural informatiX system for neuromonitoring, which combines automated electromyographic (EMG) and somatosensory evoked potential (SSEP) monitoring for lateral approach outcomes.
– EOS imaging system – full body imaging with 3D model of skeletal systems for diagnostic and surgical planning capabilities; $59 million of revenue in 2023.
· In late 2020, Alphatec launched its lateral approach to spine surgery called Prone TransPsoas (PTP) which minimizes patient repositioning, provides surgeons with optionality, and achieves spinal alignment at a higher reproducible rate. Management believes the PTP technique is in the early phase of adoption as it can be applied to multiple different spine pathologies.
· The company continues to elevate and expand its sales force while increasing sales force productivity.
· Alphatec continues to invest in new products, including 8-10 new products in 2024, to expand its total addressable market within the spine market.
· The company’s goal is to generate $1.0 billion of revenue, $180 million of adjusted EBITDA and $65 million of free cash flow in 2027.
· In April 2023, Alphatec issued 4.286 million shares at $14 per share for $57.5 million of net proceeds. In October 2023, the company issued 14.3 million shares at $10.50 per share for $145.8 million of net proceeds.
We believe Alphatec is a possible acquisition candidate for any medical company who wants to enter the spine market or to expand its spine product offering.

COMPANY OVERVIEW
Headquartered in Englewood, CO, Paragon 28 is a manufacturer and marketer of implants, biologics and systems for the treatment of a wide range of foot and ankle ailments. The company’s portfolio of nearly 80 systems consists of plates, plating systems, screws, staples, nails, orthobiologics, disposables, and instrumentation. The company markets its products using independent distributors, primarily exclusive, in the US who target orthopedic, pediatric, and trauma surgeons focused on foot and ankle procedures and surgical podiatrists. The company generated $216 million of revenue in 2023, up from $106 million in 2019. International revenue was 15% of revenue in 2023.
We highlight some key business and financial aspects:
· In 2023, revenue increased 19% to $216 million as the company continues to gain market share by launching new products and attracting new surgeon customers. The company continues to augment its sales force, which consists of 266 US sale reps at year-end.
· In any given year, the company expects to launch 5 to 10 new products and has a full pipeline with more than 30 products and systems in development.
· Its main products include the following:
- Gorilla plating system – comprehensive foot and ankle plating system with over 290 plating options and a wide variety of plate-specific screws.
– Baby Gorilla plating system – comprehensive foot and ankle specific plating system that includes 26 distinct plating styles and 92 distinct plating options with unique, customizable instrumentation.
– Silverback ankle plating system – system that offers 62 unique, low profile, anterior, lateral, and posterior plate designs, five screw diameters, and a robust offering of joint preparation instrumentation to address tibiotalar (TT), tibiotalocalcaneal (TTC), or tibiocalcaneal (TC) arthrodesis.
– Monster, Mini Monster, and Joust Beaming screw systems – screw systems used as standalone or to complement its various plating systems.
· In 2024, Paragon 28 will be launching the first module of its Smart 28 ecosystem, which utilizes analytical tools such as AI, data analytics, patient specific algorithms, 3-D modeling and other enabling technologies, to improve surgical patient outcomes.
· The company continues to expand its sales force while increasing sales force productivity.
· Management expects to generate positive adjusted EBITDA in 2024.
· In January 2022, Paragon 28 acquired Disior, a 3D analytics pre-operative planning software company, for $26.2 million and in May 2021, Paragon 28 acquired product lines of Additive Orthopedics consisting of its 3D Talus spacer for $15 million.
· In October 2021, Paragon 28 completed its IPO and issued 8.984 million shares at $16 per share for $133.7 million of net proceeds. In Jan./Feb. 2023, the company issued 4.31 million shares at $17 per share for $68.5 million of net proceeds.
We believe Paragon 28 is a possible acquisition candidate for any medical company who wants to enter or expand its foot and ankle offerings.

COMPANY OVERVIEW
Headquartered in Ponte Vedra, FL, Treace Medical Concepts is a manufacturer and marketer of implants, instruments and systems for the surgical management of bunions and related midfoot deformities. The company pioneered the Lapiplasty 3D bunion system and launched other surgical systems to correct bunion deformity. The company markets its products using more than 220 direct sales reps and more than 20 independent sales agencies targeting surgical podiatrists and orthopedic surgeons who focus on foot and ankle procedures in the US, its only market. The company generated $187 million of revenue in 2023, up from approximately $40 million in 2019.
We highlight some key business and financial aspects:
· In 2023, revenue increased 32% to $187 million as the company continues to augment its sales force, launch new products and implement its DTC awareness initiatives.
· In 2024, Treace expects to launch 10 new products and has a steady cadence of new product introductions in 2025 and beyond.
· Treace Medical pioneered the Lapiplasty 3D bunion procedure using its proprietary system. Since its launch in March 2015, more than 90,000 Lapiplasty procedures kits have been sold in the US. Benefits include correcting the 3D metatarsal alignment of the bunion, stabilizing the first TMT joint and allowing for return to weight-bearing in a walking boot. The one-year recurrence rate is 0.9% vs. double-digits for traditional bunion surgical treatment approaches.
· In addition to its Lapiplasty 3D bunion system, other products include:
- Adductoplasty system– comprehensive system for reproducible correction of metatarsus adductus deformities and osteoarthritis of the midfoot.
– Speedplate implant fixation platform – provides stability of titanium locking plate with small insertion; used for common bone fusion procedures in the foot.
– Hammertoe PEEK fixation system – system designed to treat hammertoe, claw toe and mallet toe deformities.
· The company has a five-point strategy consisting of 1) rapid focused innovation, 2) a bunion-focused direct sales channel, 3) surgeon education programs, 4) direct-to-patient education, and 5) supportive clinical evidence.
· Management expects to make progress toward adjusted breakeven EBITDA in 2024 and close to cashflow breakeven in 2025.
· In June 2023, Treace Medical acquired certain assets of RPM-3D related to pre-op surgical planning and patient specific guides for the surgical treatment of foot and ankle deformities for $20 million plus milestones.
· In April 2021, Treace Medical completed its IPO and issued 12.94 million shares @ $17 per share for $107.6 million of net proceeds. In February 2023, the company issued 5.476 million shares @ $21 per share for $107.5 million of net proceeds.
We believe Treace Medical is a possible acquisition candidate for any medical company who wants to enter or expand its bunion and foot offerings.

COMPANY OVERVIEW
Headquartered in Warsaw, IN, OrthoPediatrics is a manufacturer and marketer of implants and products for pediatric patients with orthopedic conditions. The company was founded in November 2007 and went public in October 2017 at $13.00 per share. The company’s products include its trauma and deformity product line (72% of revenue), its scoliosis systems (25% of revenue) and sports medicine products (3% of revenue). The company has the broadest pediatric specific orthopedic offering with 53 surgical implant systems. The company markets its products in the US using 40 sales agencies employing 200 sales reps, exclusive to the company. The company generated $149 million of revenue in 2023. International revenue was 25% of total revenue in 2023.
OrthoPediatrics is an orthopedic implant company focused on pediatric patients. We highlight some key business and financial aspects:
· In 2023, revenue increased 22% to $149 million with $5.3 million from acquisitions, or 4%.
· Revenue was driven by increased sales of Pega products, PNP femur, Cannulated screws, Orthex xystem, RESPONSE systems, ApiFix and 7D flash surgical navigation system.
· The company has grown revenue at a consistent 20%+ rate since inception.
· Its main products include the following:
– Trauma and deformity (~72% of revenue) – More than 7,000 implants and bone fixation devices for the femur, tibia and upper and lower extremities including cannulated screws, locking cannulated blade, locking proximal femur, PediFlex flexible nailing system, PediNail intramedullary nail, PediPlates, PediLoc, ACL reconstruction system, and others.
– Scoliosis (~25% of revenue) –Includes RESPONSE systems for treating spinal deformity, ApiFix system for adolescent idiopathic scoliosis, BandLoc 5.5mm/6.0mm sub-laminar banding system, FIREFLY® pedicle screw navigation guides, and 7D flash surgical navigation image guidance system.
– Sports medicine (3% of revenue) – ACL and MPFL Reconstruction system and Telos.
· In the US, the company has 40 sales agencies employing 200 sales reps who are present in the operating room with the surgeon customer; the company provides extensive training to these sales reps.
· Outside the US, the company has 70 stocking distributors and 14 independent sales agencies in over 70 countries. It has direct sales programs in most of the European countries, Australia, New Zealand, and Canada.
· In 2023 and early 2024, the company made two acquisitions: 1) Medtech Concepts, a pre-commercial enabling tech platform for perioperative efficiency, for $15.3 million in cash and stock, and 2) Boston Brace, a manufacturer of pediatric orthotic and prosthetic devices, for $22 million in cash.
· In 2023, the company invested ~$15 million in instrument sets to support its growth in existing and new markets and expects to invest less than $20 million in 2024.
· Management expects $8-9 million adjusted EBITDA for 2024.
We believe OrthoPediatrics is a possible acquisition candidate for any medical company who wants to expand its pediatric product offering.

COMPANY OVERVIEW
Headquartered in Lewisville, TX, Orthofix is a global spine and orthopedics company with comprehensive offerings in spine and biologics, bone growth stimulation, and specialty trauma/ fixation. The company increased its product portfolio and scale with the merger with Seaspine in January 2023. The company’s segments include spine and biologics (56% of sales), bone growth stimulation products (29% of sales), and specialized orthopedics/ fixations products (15% of sales). The company generated $747 million of revenue in 2023. International sales represented 17% of total sales in 2023.
We highlight some key business and financial aspects about Orthofix:
· Fifth largest spine company in the world with its combined spine implants, biologics and bone growth stimulation revenue.
· 2023 was the first year of integration for Orthofix and Seaspine, which will continue through 2025. In 2023, Orthofix realized $32 million of cost synergies and is progressing toward its $50 million target by year-end 2025.
· In January 2024, Orthofix named Massimo Calafiore as its new CEO and Julie Andrews as its new CFO; both have extensive experience in orthopedics and medical devices.
· In 2023, Orthofix’s revenue grew 8% pro-forma in constant currency to $747 million with 13.5% growth in bone growth stimulation, 6% growth in spine/ biologics and 5% growth in global orthopedics.
· The company’s segments include the following products:
– Spine/ biologics solutions – repair and regenerative products to treat a variety of spinal conditions. Key products various pedicle screw systems, M6 artificial cervical discs, portfolio of products for ALIF, PLIF, TLIF and LLIF procedures including interbody spacers and access systems, and 7D flash navigation system and products for other spinal procedures; Biologics include Trinity Elite™ tissue forms, demineralized bone fibers products such as Strand(Plus) and FiberFuse, Ballast products.
– Bone growth stimulation therapies – Portfolio of devices for enhancing bone fusion that utilize Orthofix’s patented pulsed electromagnetic (PEMF) technology; FDA-approved Class III medical devices indicated as an adjunctive treatment to enhance fusion success in cervical and lumbar spine, nonunion fractures and fresh fractures – CervicalStim, SpinalStim, PhysioStim, and AccelStim.
– Specialized trauma/extremity fixation – Portfolio of repair and regenerative products that allow physicians to successfully treat a variety of orthopedic conditions including products used in fracture repair, deformity correction and bone reconstruction including TrueLok external fixation system, FITBONE limb lengthening system, Galaxy fixation system and pediatric portfolio.
· Management is focused on growing its business segments in a profitable manner.
· Management expects to achieve positive cash flow in the fourth quarter of 2024.
· As of December 31, 2023, Orthofix had $38 million of cash and $94 million of debt on its balance sheet.
With its merger with SeaSpine, Orthofix will be able to compete more effectively in the global spine market.

COMPANY OVERVIEW
Headquartered in Santa Clara, CA, Si-Bone is a manufacturer and marketer of the iFuse implant system for minimally invasive surgical treatment of the sacroiliac jointa (SIJ) in the lower back. The company was founded in 2008 and went public in October 2018 at $15.00 per share. The company’s products include its iFuse family of implant systems and enabling technology solutions for the surgical procedure of the sacroiliac joint. The company markets its products using more than 150 field sales reps and clinical support specialists in the US. Outside the US, the company has 14 direct sales reps and works with 31 exclusive distributors to market its products in 38 countries. The company generated $139 million of revenue in 2023. International revenue was 6% of total sales in 2023.
We highlight some key business and financial aspects:
· In 2023, revenue increased 30.5% to $138.9 million due to increased volume with increased productivity for its US sales organization and growing base of active surgeons.
· The iFuse family of implant systems includes a series of patented triangular implants, instruments and diagnostic and surgical techniques to perform the surgical treatment of the sacroiliac joint, sacropelvic fixation and pelvic fractures.
· The US market opportunity for sacroiliac joint treatment, sacropelvic fixation and pelvic fractures is approximately 470,000 patients per year or $3.0 billion opportunity; Si-Bone’s implants were used in more than 15,000 procedures in 2023 in the US.
· According to Si-Bone, iFuse is the market leader with over 95,000 worldwide procedures since inception.
· Benefits of the iFuse-3D implant system include: a) significant decrease in mean pain and disability improvement scores; b) 95% patient satisfaction rate (3.5% revision rate at 4 years); c) significant reduction in opioid use post surgery; d) over 300 US million lives covered by insurance; and e) long-term five year data.
· There are more than 125+ peer reviewed published papers and 6-year long-term data on the iFuse system, which is the most for any SIJ implant.
· In early 2024, Si-Bone began its launch of its iFuse INTRA, an allograft product, which provides intra-articular stabilization and fusion from a posterior approach; Si-Bone expects to launch more products in 2024.
· Management is focused on increasing its sales reps and its sales coverage in the US along with medical training and education; currently has 82 field sales reps and 69 clinical support specialists in the US.
· iFuse implant averages close to $10,000 with 78% gross margins expected in 2024 with low-to-mid single digits price declines.
· The company has 59 US and 18 international issued patents covering the iFuse through December 2025 and other iFuse patents through 2040.
· With its strong sales growth and operating leverage, Si-Bone is making progress towards its breakeven EBITDA goal, which we believe is achievable in 2025 or 2026.
· In May 2023, Si-Bone issued 4.1 million shares @ $22 for net proceeds of $83.7 million.
· As of December 31, 2023, Si-Bone had $166 million of cash and $36 million of debt on its balance sheet.
We believe Si-Bone is a possible acquisition candidate with its unique iFuse family of implants.
a) The sacroiliac joint connects the base of the spine to the hip joint.
Jennie Tsai © Gabelli Funds 2024
(914) 921-8436
jtsai@gabelli.com
ONE CORPORATE CENTER RYE, NY 10580 Gabelli Funds TEL (914) 921-5000